Discovery
Luminescence is often weakened or quenched at high concentrations, a phenomenon widely known as ‘concentration quenching’ (Figure 1, A). A main cause for the quenching process is mechanistically associated with the ‘formation of aggregates’, which is probably why the concentration quenching effect has frequently been referred to as ‘aggregation-caused quenching’ (ACQ).
In 2001, we discovered an uncommon luminogen system, in which aggregation worked constructively, rather than destructively as in the conventional systems.20,21 We found that a series of silole derivatives were non-emissive in dilute solutions but became highly luminescent when their molecules were aggregated in concentrated solutions or cast into solid films (Figure 1, B). Since the light emission was induced by aggregate formation, we termed the process ‘aggregation-induced emission’ (AIE).

Figure 1. Comparison of ACQ and AIE
Representative publications:
[Reviews: Aggregation-Induced Emission Chem. Soc. Rev. 2011, 40, in press; Aggregation-Induced Emission: Phenomenon,
Mechanism and Applications Chem. Commun. 2009, 4332]
Mechanism
In a dilute solution, six phenyl rotors in an HPS molecule undergo dynamic intramolecular rotations against its silacyclopentadiene or silole stator, which non-radiatively annihilates its excited state and renders its molecule non-luminescent. In the aggregates, the HPS molecules cannot pack through a pi-pi stacking process due to its propeller shape, while the intramolecular rotations of its aryl rotors are greatly restricted owing to the physical constraint. This restriction of intramolecular rotations (RIR) blocks the non-radiative pathway and opens up the radiative channel. As a result, the HPS molecules become emissive in the aggregate state.
According to our proposed mechanism, the RIR process is responsible for the AIE effect. We have conducted a number of control experiments (decreasing temperature, increasing viscosity, applying pressure, etc.) to externally activate the RIR process. We have also used covalent bonds to fasten the aryl rotors to internally set off the RIR process at the molecular level. In response to both the external and internal controls, the luminogens become emissive, thus offering experimental evidence to support our mechanistic hypothesis.

Figure 2. Mechanistic study of AIE phenomenon
AIE system
Guided by the mechanistic understanding, we synthesized a large number of luminogens with propeller-shaped molecular structures. We systematically studied their PL behaviour with the aim of developing new AIE systems and gaining more insight into AIE processes.

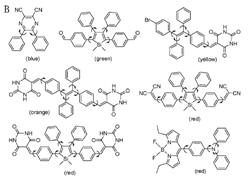
Figure 3. Examples of (A) pure hydrocarbon and (B) heteroatom-containing AIE fluorophores developed in our lab
Technical Applicaions
New concepts lead to new applications. In principle, the AIE effect can be utilized to do useful work wherever the RIR process is involved. The possibilities are therefore plentiful, limited probably only by our imagination. Here are some typical selective applications of our AIE chromophores.

Figure 4. Demonstration of technical applications of AIE chromophores
I.Chemo-sensors
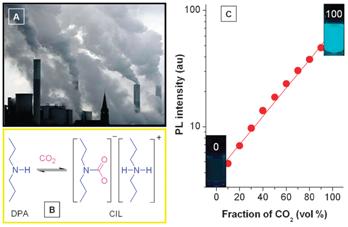
Figure 5. Working scheme of CO2 sensor
Our groups have developed an alternative scheme based on AIE luminogens for selective detection and quantitative assay of CO2 gas. In this scheme, HPS is dissolved in an amine solvent such as dipropylamine (DPA), giving a non-luminescent solution. When CO2 gas is bubbled through the solution, it reacts with DPA to yield a carbamate ionic liquid (CIL; Figure 5B). The high viscosity and polarity of CIL slow down the torsional motions of the aryl rotors and induce the HPS molecules to aggregate. As the RIR process is set off, the HPS emission is turned on. With more CO2 gas bubbled through the solution, more CIL is formed. The PL intensity thus mirrors the CO2 level (Figure 5C), which provides an inexpensive and visually discernable method for quantitative detection CO2 gas with applications as diverse as predicting volcanic eruptions and signalling danger in the environment.
Representative publication:
[J. Am. Chem. Soc. 2010, 132, 13951]

Figure 6. Explosive detection using AEE hyperbranched polymer
An example of explosive detection system using an AEE active hyperbranched polymer as the probe is shown in Figure 6. The fluorescence of the nanoaggregates of the polymer suspended in an aqueous mixture is progressively attenuated with the sequential addition of PA. The emission quenching can be clearly distinguished at a PA level ([PA]) as low as 1 ppm. The I-[PA] plot gives a swiftly upward-bending curve rather than a linear line, suggestive of a superamplification effect (Figure 6C). The quenching constant is 1.35 X 105 L mol-1, much higher than those of other explosive sensors. The 3D topological structures of the hyperbranched polymer and its aggregates provide many internal cavities for the explosive molecules and various diffusion paths for the excited states, leading to the extra-ordinary sensing performance. The superamplification effect has been observed in many AIE and AEE nanoaggregate systems, proving that it is a common feature for the AIE- and AEE-based sensing systems.
Representative publication:
[Polym. Chem. 2010, 1, 426]
II. Bio-sensors


Figure 7. Specific Detection of D-Glucose by a Tetraphenylethene-Based Fluorescent Sensor
A conceptually new “light-up” biosensor with a high specificity for D-glucose (Glu) in aqueous media has been developed. The emission from a tetraphenylethene (TPE)-cored diboronic acid (1) was greatly boosted when the fluorogen was oligomerized with Glu because of restriction of the intramolecular rotations of the aryl rotors of TPE by formation of the oligomer. Little change in the light emission was observed when 1 was mixed with D-fructose, D-galactose, or D-mannose, as these saccharides are unable to oligomerize with the fluorogen.
Representative publication:
[J. Am. Chem. Soc. 2011, 133, 660]

Figure 8. Fluorescence ‘turn-on’ probe for specific thiol detection
A probe for thiol detection was developed by modifying TPE with a maleimide (MI) group. While TPE–MI adduct 70a is nonemissive in both solution and solid states due to the photoinduced electron transfer from TPE to MI unit, its thiolated derivative 70b shows AIE effect (Figure 8A). In other words, 70a is a ‘caged’ AIE luminogen. Its aggregates deposited on TLC plates can be used to differentiate cysteine from other amino acids through the thiol - ene click reaction (Figure 8B). Because the click reaction is very fast and efficient at room temperature, 70a can also be used for labelling proteins carrying cysteine residues in the physiological media and in the poly(acrylamide) gel electrophoresis (PAGE) assays.
Representative publication:
[Chem. Eur. J. 2010, 16, 8433]
III. Cell imaging
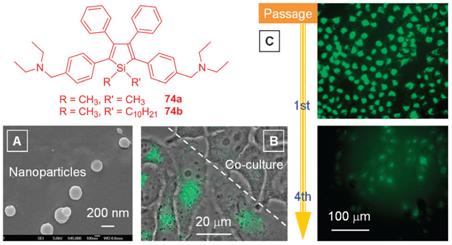
Figure 9. Cytophilic Fluorescent Bioprobes for Long-Term Cell Tracking
Because of their good water miscibility, ionic luminophores have often been used for cell imaging applications. However, at high concentrations, their charges can perturb membrane potential and cellular physiology. On the other hand, at low concentrations, the small numbers of the luminophores that have entered into the cellular interiors can be easily photobleached in the imaging process. During the cell division process and when the confluent cells are passaged, the intracellular dyes often diffuse back to the extracellular media owing to the existence of the concentration gradient. This leads to a decrease in the emission of the stained cells and a concurrent increase in the solution (or background) emission as well as a random staining in a cell co-culture system.
Aminated silole derivatives 74 are non-ionic luminogens that spontaneously aggregate into nanoparticles in aqueous media (Figure 9A). The nanoparticles are cytocompatible and can be internalized via an endocytosis process. Due to their AIE nature and electrical neutrality, the luminogens can be used in high concentrations in cell imaging processes. The nanoaggregates indelibly stain the HeLa cells because the internalized particles are difficult to escape from the cellular compartments. The strong retention of the nanoparticles in the HeLa cells prevents the cross-staining between different cell lines and enables the fluorescence cell differentiation (Figure 9B). The internalized particles are withheld inside the HeLa cells so robustly that they remain visible for a long period (up to four passages), permitting visual monitoring of the growth of a specific type of cell line (Figure 9C).
Representative publication:
[Adv. Mater. 2011, 23, 3298]
IV. Protein conformation study

Figure 10. Quantitation, Visualization, and Monitoring of Conformational Transitions of Human Serum Albumin (HSA)
For a protein to perform its biological functions, it must assume a specific chain conformation. The development of luminescent tools for studying protein conformations is thus of obvious importance. Investigation of global stability of protein chains in the presence of denaturants has attracted much interest, as it offers mechanistic insights into protein (un)folding processes. Though intermediate states are often involved in the (un)folding processes of many proteins, they are difficult to detect due to the lack of appropriate probes.
We developed an emission turn-on sensor for human serum albumin (HSA) using a sulfonated TPE (73) as probe. The emission of 73 in an aqueous buffer was switched on in the presence of HSA. The HSA-triggered emission allowed 73 to be used as a visualization agent in the PAGE assays. HSA is a main protein component of the blood plasma. Its hydrophobic regions are capable of binding with insoluble endo- and exogenous compounds like fatty acids and drugs. The hydrophobicity of the phenyl rings of 73 prompted the luminogenic molecules to enter into and aggregate inside the hydrophobic cavities or pockets of the folding structures of HSA chains (Fig. 34). Utilizing the AIE nature of 73 and the Forster resonance energy transfer (FRET) from the intrinsic fluorescence of HSA at 350 nm to the AIE emission of 73 at 470 nm, the unfolding trajectory of HSA chains induced by denaturant of guanidine hydrochloride (GdnHCl) was traced, which revealed a three-step transition with a stable moltenglobule intermediate involved in the unfolding process.
Representative publication:
[Anal. Chem. 2010, 82, 7035]
V. Strategy to design highly efficient light emitters in the solid state

Figure 12. A new strategy for solving the ACQ problem: attachment of AIE groups to ACQ units results in the formation of new luminogens with high solid-state emission efficiencies.
The flat molecule of anthracene (59) is ACQ active. It is transformed to an AIE luminogen by decorating it with a TPE unit. The solution of adduct 60 is non-emissive but its aggregates luminesce very efficiently (ΦF,a = 100%). This strategy works equally well for other ACQ molecules. Embellishing an ACQ-active TPA core with multiple AIE-active TPE peripherals, for example, affords adduct 62 with combined advantages of the two components: holetransport capability (TPA) and AIE activity (TPE). Thanks to the electronic conjugation of the TPE component, the excellent hole-transporting property of the TPA component is well retained in 62, experimentally verifying the rationale and feasibility of our new strategy.
The conventional approaches mitigate the ACQ effect but bring about new problems. In contrast, our new approach solves the ACQ problem without causing undesired sideeffects. The examples discussed above show that embossing ACQ luminophores with TPE units is a general and versatile strategy for eliminating ACQ effect and generating new AIE luminogens. Our new strategy takes advantage of the natural process of aggregate formation and therefore does not suffer from temporal and spatial instabilities.
Representative publication:
[Adv. Mater. 2010, 22, 2159]
VI. OLED

Figure 11. Photographs of cyan, orange and white light-emitting EL devices using pure 64 and 66 and a mixture of them (64/66) as emitting layers, respectively.
To attain full-colour display, AIE luminogens with emission colours covering the whole range of visible lights have been designed and prepared. Some examples of the luminogens are shown in. Many OLEDs using the AIE luminogens as the emitting layers have been fabricated.
Representative publication:
[Curr. Org. Chem. 2010, 14, 2109]
